- diagnosing, treating, mitigating or preventing a disease, disorder or abnormal physical state, or any of their symptoms, in human beings or animals;
- restoring, modifying or correcting the body structure of human beings or animals or the functioning of any part of the bodies of human beings or animals;
- diagnosing pregnancy in human beings or animals;
- caring for human beings or animals during pregnancy or at or after the birth of the offspring, including caring for the offspring; and
- preventing conception in human beings or animals.
However, it does not include such an instrument, apparatus, contrivance or article, or a component, part or accessory of any of them, that does any of the actions referred to in paragraphs (a) to (e) solely by pharmacological, immunological or metabolic means or solely by chemical means in or on the body of a human being or animal.
Medical Device (as defined in section 1 of the Medical Devices Regulations) - refers to a device within the meaning of the Act, but does not include any device that is intended for use on animals.
2. What is required to sell a medical device in Canada?
Health Canada issues two types of licences for medical devices:
- Medical Device Licence (MDL) - a licence issued to manufacturers authorizing them to import or sell their Class II, III or IV medical devices in Canada. For more information on how to obtain an MDL, see Guidance Document: How to Complete the Application for a New Medical Device Licence.
- Medical Device Establishment Licence (MDEL) - a licence issued to Class I manufacturers, as well as importers or distributors, of all four device classes to permit importation or distribution (sale) of a medical device in Canada. For more information on how to obtain an MDEL, see Guidance on Medical Device Establishment Licensing (GUI-0016).
3. What is a Medical Device Establishment Licence (MDEL)?
A Medical Device Establishment Licence (MDEL) is issued for the activities of manufacturing (class I), importing or distributing (selling) medical devices for human use in Canada. An MDEL is issued by Health Canada's Regulatory Operations and Enforcement Branch (ROEB), based on an attestation that the establishment meets all of Health Canada's MDEL regulatory requirements.
During an inspection by Health Canada, MDEL holders must demonstrate to Health Canada that they meet the regulatory requirements they attested to having in place (e.g. documented procedures in place) related to the medical devices that they manufacture, import or distribute (sell) in Canada.
- offer for sale, expose for sale or have in possession for sale, or distribute to one or more persons, whether or not the distribution is made for consideration; and
- lease, offer for lease, expose for lease or have in possession for lease.
4. Who requires a Medical Device Establishment Licence (MDEL)?
A Medical Device Establishment Licence (MDEL) is required by Class I manufacturers, as well as importers or distributors of all four device classes to permit importation or distribution (sale) of a medical device in Canada.
5. Who is exempt from holding a Medical Device Establishment Licence (MDEL)?
The following are exempt from holding a Medical Device Establishment Licence (MDEL) under the Medical Devices Regulations to import into, or sell a medical device in Canada:
- any person who imports a medical device for his/her own personal use
- retailers, including:
- companies that sell medical devices to the end-user (ultimate consumer or end user) for their own personal use; and
- Canadian manufacturers of Class I medical devices who sell their devices solely to ultimate consumers or end users.
- medical devices for which they hold a valid Medical Device Licence (MDL)
- medical devices subject to parts 2 and 3 of the Medical Devices Regulations* to be exempt, the manufacturer cannot import or sell medical devices manufactured by other companies.
- custom-made devices;
- for special access; and
- medical devices for investigational testing involving human subjects (clinical trials).
- Section 37 applies to medical devices that, although manufactured in Canada, are not intended to be sold for use in Canada.
- Companies intending to invoke section 37 of the Food and Drugs Act related to medical devices must also meet the relevant requirements under the Medical Devices Regulations.
- To be exempt, warehouses must not buy, sell or consign medical devices (see definition of warehouse in MDEL application GUI-0016, Appendix A - Glossary).
6. What should I know before filing a Medical Device Establishment Licence (MDEL) application?
Since there are no provisions for refunds of fees, once an application has been submitted to Health Canada for review, it is your responsibility to confirm the following before filing an application:
- determine the classification of your device (see question 7 . How should I confirm the classification of a device before applying for a Medical Device Establishment Licence? for more information);
- determine the appropriate licence fee payable to Health Canada (see question 9 . Is there a fee for a Medical Device Establishment Licence (MDEL)? for more information);
- complete and submit an MDEL application (see question 8 . How do I apply for a Medical Device Establishment Licence (MDEL)? for more information); and
- provide accurate information on the MDEL application forms.
7. How should I confirm the classification of a device before applying for a Medical Device Establishment Licence?
Information regarding the classification of a device is provided in the following documents:
- Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices
- Guidance Document - Guidance on the Risk-based Classification System for Non-In Vitro Diagnostic Devices (non-IVDDs)
- Guidance for the Risk-based Classification System for In Vitro Diagnostic Devices (IVDDs)
You may also contact the Medical Devices Directorate for questions about medical devices including classification, labelling, clinical trials and obtaining a medical device licence:
- E-mail: meddevices-instrumentsmed@hc-sc.gc.ca
- Telephone: 613-957-7285 613-957-7285
Applying for a Medical Device Establishment Licence (MDEL)
8. How do I apply for a Medical Device Establishment Licence (MDEL)?
Step 1 Review Guidance on Medical Device Establishment Licensing (GUI-0016). Step 2 Complete the Medical Device Establishment License Application (FRM-0292) Step 3
Note: Please do not submit duplicate applications.
9. Is there a fee for a Medical Device Establishment Licence (MDEL)?
Yes. There is a fee for the review of the following Medical Device Establishment Licence (MDEL) applications:
- New applications;
- Annual Licence Review (ALR) applications; and
- Reinstatement applications.
10. Do I need to apply for Small Business status prior to submitting my New MDEL application?
Yes. To be eligible for the small business fee mitigation, applicants must apply for small business status before submitting their Medical Device Establishment Licence (MDEL) application, otherwise the full fee will be charged. Please visit Health Canada's Funding and Fees webpage for more information on how to apply for small business status.
11. How long does it take Health Canada to review a Medical Device Establishment Licence (MDEL) application?
The performance standard to issue a decision is 120 calendar days from the day a complete Medical Device Establishment Licence (MDEL) application is received. This performance standard applies to the following application types:
- New applications; and
- Annual Licence Review (ALR) applications
- Reinstatement applications
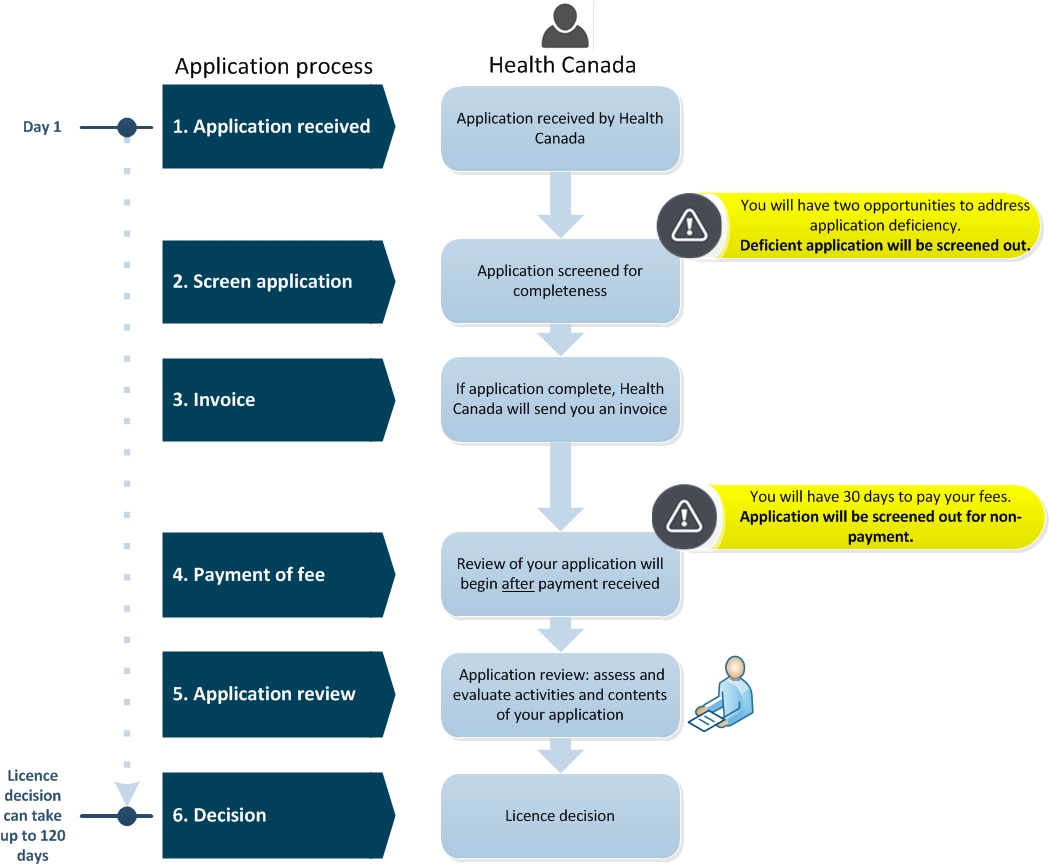
Diagram 1. Medical Device Establishment Licence (MDEL) application screening and review process - Text description
This flowchart is divided into two sections; the first left section is entitled, "Application Process" and the second right section is entitled, "Health Canada". The left section indicates the steps required in the application process and the right section describes the tasks taken by Health Canada.
Step 1 is entitled "Application received". In this step, Day 1, Health Canada received the MDEL application.
Step 2 is entitled "Screen application". Health Canada screens the application for completeness. Between Step 1 and 2, there is an important point to note, that the applicant will have two opportunities to address application deficiencies. After while, the deficient application will be screened out.
Step 3 is entitled "Invoice". If the application is complete, Health Canada will send you an invoice.
Step 4 is entitled "Payment of fee". Health Canada will begin the review your application after payment is received. There is an important point to note, you will have 30 calendar days to pay your invoice fees. Applications will be screened out for non-payment.
Step 5 is entitled "Application review". Application review includes the assessment and evaluation activities and content of your application by Health Canada.
Step 6 is entitled "Decision". Health Canada will issue a licence decision. This licence decision can take up to 120 calendar days.
This concludes the flowchart.
12. When will I receive acknowledgement from Health Canada regarding my Medical Device Establishment Licence (MDEL) application?
You will receive acknowledgement from Health Canada regarding your Medical Device Establishment Licence (MDEL) application as soon as it is received and screened for completeness. MDEL applications are screened to verify for completeness against the following criteria:
- application file is not corrupt and not password protected (we do not accept password protected.PDF documents)
- all relevant sections of the application form are complete and signed
- all indicated documents and emails have been included
- verification / confirmation of requested changes
If Health Canada deems the application complete, applicants will be notified via email indicating that the application has been accepted. An invoice with the applicable fees will also be included.
13. How do I check the status of my Medical Device Establishment Licence (MDEL) application?
Health Canada will respond to status update requests if the inquiries identify that:
- You have not received a notice that your application has been accepted for further review within 30 calendar days of the date that it was received by our office
- It has been 120 calendar days since you submitted your application and Health Canada has not notified you of our decision about your application
For both situations, you may request a status update at: mdel.questions.leim@hc-sc.gc.ca.
We will contact applicants via email during the licensing process should we have questions or require additional information.
14. What is the signed attestations portion of the Medical Device Establishment Licence (MDEL) application form and who is required to sign that section of the MDEL application?
The attestation from a senior official of an establishment provides assurance that medical devices manufactured, sold or imported into Canada meet the safety requirements set out in the Medical Devices Regulations, and that documented procedures are in place to protect Canadians, should a problem arise.
A senior official listed on a Medical Device Establishment Licence (MDEL) application is the person who has direct knowledge of the documented procedures in place, as confirmed by signing the attestations in section 7 of the MDEL application FRM-0292.
A senior official from your establishment must complete the attestations provided under paragraphs 45(g), (h), (h.1) and (i) of the Medical Devices
Regulations, as applicable, based on the activities conducted by your establishment.The name of the senior official associated with an MDEL is published in Health Canada's Medical devices establishment licence listing along with other posted information about establishments.
15. Do I still need to hold a Medical Device Establishment Licence (MDEL) if I only sell one medical device a year?
Yes, any person who manufactures (class I), imports or sells a medical device for human use in Canada requires a Medical Device Establishment Licence (MDEL). The requirement to hold an MDEL does not depend on the quantity sold per year.
16. What activities should I list in my Medical Device Establishment Licence (MDEL) application?
You should list all activities that apply, based on the following:
- Class I Manufacturer: if your company name is the only company listed on the label or if your company is identified on the label as the manufacturer of a Class I Device. Class I manufacturers may or may not be located in Canada.
- Importer: if you are a company in Canada responsible for bringing medical devices into Canada.
- Distributor: if you are selling medical devices received from a manufacturer or supplier in Canada or if you are a company located outside of Canada selling medical devices in Canada other than the devices for which you are not the legal manufacturer.
17. What do I list as a site?
A site is any additional building that is used by the Medical Device Establishment Licence (MDEL) holder (establishment) for keeping and carrying out the documented procedures attested to in paragraphs 45(g), (h), (h.1) and (i) of the Medical Devices Regulations (FRM-0292).
If the site listed is not under the same legal entity, it is the responsibility of the licence holder to ensure that the site(s) listed in section 4 of their MDEL application has the applicable documented procedures in place and that inspectors are able to verify compliance without impediment.
You may list a building under section 4 (site), including a warehouse, as long as the following conditions are satisfied:
- It is a physical location where attested procedures are conducted (a P.O. Box is not an acceptable site address).
- You must indicate at least one site in section 4 where the documented procedures are stored. If there is more than one site where the documented procedures take place, you must indicate all these sites in section 4 and the specific documented procedure(s) at each site listed.
- A site must be in the same country as the main address listed on the establishment licence. For example, if the main address for the MDEL (the establishment licence address) is located in Canada, then only sites within Canada can be listed in section 4, or if the establishment licence address is located in the USA, then only sites within the USA can be listed in section 4.
18. Where do I find the company ID? Is it the same as my registered business number?
The company ID is not your business number. A company ID is a six digit number assigned by Health Canada.
If you import and/or distribute Class II, III and IV medical devices you can find the company ID of the manufacturer on the Medical devices active licences website. This website lists all manufacturers of class II, III and IV medical devices that currently hold an active medical device licence (MDALL).
If you hold a Medical Device Establishment Licence (MDEL) to import, distribute or manufacture Class I medical devices, you can find your company ID on the Medical Devices Establishment Licence Listing.
19. What if my Medical Device Establishment Licence (MDEL) application is incomplete?
During the review of a Medical Device Establishment Licence (MDEL) application, Health Canada will contact the applicant by email if it is determined that any information necessary for the issuance of a licence is missing. The applicant will be provided two opportunities to submit the missing information.
Failure to respond to a deficiency notice will result in the rejection of the application.
20. What is a deficiency notice?
A deficiency notice is sent when an application cannot be further processed by Health Canada because it does not meet the regulatory requirements or the intent/scope of the application is not clear. The applicant is provided an opportunity to submit the missing or incomplete information in order to avoid receiving a rejection of the application within 30 business days.
21. How will the deficiency notice impact the overall application review time (120 days)?
The performance standard to issue a decision is 120 calendar days from the day a complete Medical Device Establishment Licence (MDEL) application is received. This performance standard applies to the following application types:
- New application; and
- Annual Licence Review (ALR) application
- Reinstatement application
Health Canada uses a "clock" to measure performance against the 120 calendar day performance standard:
- The clock starts on the date when Health Canada receives your complete application.
- In cases where the application requirements listed in the Food and Drugs Act or its Medical Devices Regulations are not met, a deficiency notice is issued to the applicant (via email) and the clock is paused.
- When Health Canada has received a response to a deficiency notice, the clock will be restarted.
Amendments and notifications
22. What is a notification? What is an amendment? How do I submit these changes to my Medical Device Establishment Licence (MDEL)?
Notification. You must submit a notification to Health Canada within 15 calendar days of, as per section 48 of the Medical Devices Regulations:
- a change in the name or address of your establishment; and
- a change in the information of the establishment representative associated with your Medical Device Establishment Licence (MDEL) application including that person's name, title and telephone number.
Notify Health Canada by submitting a revised FRM-0292 and sending it via email to mdel.application.leim@hc-sc.gc.ca.
Amendment. You can submit changes to your existing MDEL (for example, list of manufacturers, a change in activity or class of device), at any time.
- mid-December to March 31 - you can submit your changes as part of your Annual Licence Review (ALR) application.
- April 1 to mid-December - you can submit your changes using FRM-0292.
23. If my company intends to add another activity or class of device to our business line, do I need to inform Health Canada?
Yes, an amendment application is required prior to beginning new activities, including adding different classes of medical devices.
24. Is there a fee for an amendment or notification?
No. There is no fee for amending or notifying a Medical Device Establishment Licence (MDEL).
Annual licence review
25. What is an Annual Licence Review?
Under the Medical Devices Regulations, all active Medical Device Establishment Licence (MDEL) holders must submit an application for Annual Licence Review (ALR), prior to April 1 of each year. The purpose of an ALR is to ensure continued compliance with regulatory requirements and to maintain up-to-date information. You must submit this application annually and pay the fee upon receiving an invoice even if there are no changes to your licence.
As a courtesy, Health Canada sends an ALR application package to all active MDEL holders at the end of December of each year. However, it is your responsibility to ensure a complete ALR application is received by Health Canada well before April 1 of each year. If you do not receive your ALR package by mid-January, contact Health Canada.
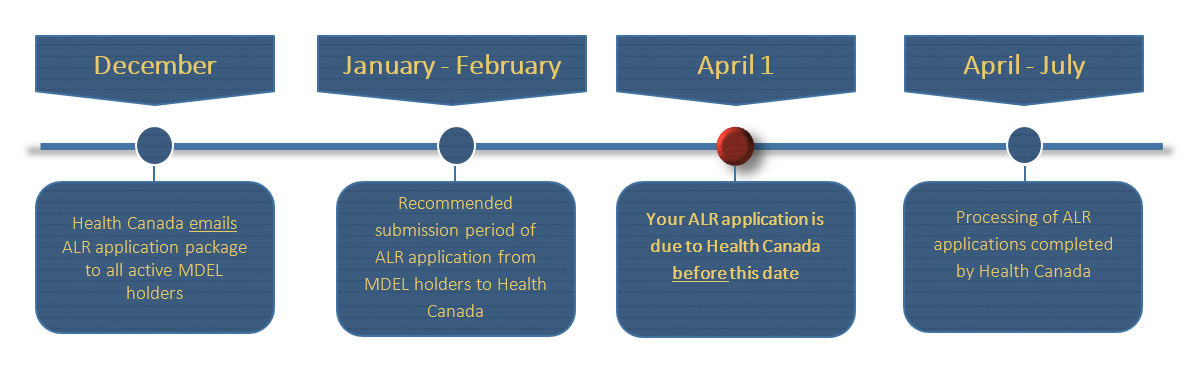
Diagram 2. Annual Licence Review timeline - Text description
This flowchart is divided into two sections; the upper section is a monthly timeline and lower section indicates the requirements at each time point noted in the box above.
In December, Health Canada emails Annual Licence Review application package to all active MDEL holders.
In January to February, the timeline that is the recommended submission period of Annual Licence Review application from MDEL holders to Health Canada.
April 1, is the due date for your Annual Licence Review application to Health Canada.
In April to July, the timeline for the processing of ALR applications completed by Health Canada.
This concludes the flowchart.
26. Is there a fee for Annual Licence Review?
Yes. There is a fee for the review of the Annual Licence Review (ALR) application. The fee is payable within 30 calendar days from the date of invoice. Interest on overdue accounts begins to accrue 30 days from the date of invoice issuance.
If the Medical Device Establishment Licence (MDEL) fees have not been paid, Health Canada has the authority to withhold services, approvals, rights and/or privileges.
The applicable fee is specified in the Fees in Respect of Drugs and Medical Devices Order. As of April 1, 2020, new fees will be in effect. These fees will also increase annually to keep up with inflation.
For more information on MDEL fees, please see:
- Guidance document - Fees for the Review of Medical Device Establishment Licence Applications
- How to Pay Your Establishment License Fees
Some establishments may be eligible for small business fee mitigation, where the full fee is reduced by 25%. Please see questions 46 . Can my Medical Device Establishment Licence (MDEL) fee be reduced? -53 . I am a new applicant. Do I need to pay when applying? for more information about fees.
27. Do I still have to submit an application for Annual Licence Review, even if there are no changes to my Medical Device Establishment Licence (MDEL)?
Yes. An Annual Licence Review application must be submitted prior to April 1 of each year even if there are no changes to the content of your Medical Device Establishment Licence (MDEL) application. You are required to pay the applicable fees once you receive an invoice.
28. What is the deadline for submitting an Annual Licence Review application?
Annual Licence Review applications must be submitted prior to April 1 of each year.
29. What if I do not submit my Annual Licence Review application before April 1?
If you do not submit an Annual Licence Review (ALR) application before April 1 of each year, Health Canada will cancel your Medical Device Establishment Licence (MDEL) starting on April 1, at which point you will no longer be authorized to conduct licensable activities. To resume conducting licensable activities you will need to apply for a new MDEL. Please see questions 8 . How do I apply for a Medical Device Establishment Licence (MDEL)? -10 . How long does it take Health Canada to review a Medical Device Establishment Licence (MDEL) application? on how to apply for an MDEL.
30. May I submit my Annual Licence Review by email?
Yes. Please submit the Annual Licence Review application to: mdel.application.leim@hc-sc.gc.ca.
31. What is the completion time for an Annual Licence Review?
The performance standard for the review of an Annual Licence Review (ALR) application is 120 calendar days from the day it is received by Health Canada.
Health Canada uses a "clock" to measure performance against the 120 calendar day performance standard.
- The clock starts on the date when Health Canada receives your Annual Licence Review application.
- When a deficiency is identified within your Annual Licence Review application, a deficiency notice is issued and the clock is paused.
- The clock is restarted once HC receives a response to the deficiency notice.
32. Will my current licence be valid during the Annual Licence Review?
Yes, your Medical Device Establishment Licence (MDEL) will continue to be valid as long as you submit your application for Annual Licence Review (ALR) before April 1 of each year, and pay the associated fee.
33. How will I know when my Annual Licence Review is completed?
After you submit you Annual Licence Review (ALR) application to Health Canada, you will receive the following communication to inform you of the licence process:
- Health Canada will send you a confirmation by email that your application has been received and entered in our system;
- You will then receive an invoice from Health Canada for the review of your ALR application;
- You must pay the fee within the timeline indicated in the invoice; and
- After you pay the fee, Health Canada will review your application and send you an email to confirm its completion.
You will be contacted if the application is incomplete or if concerns are raised that may affect the issuance of the licence. Please see questions 19 . What is a deficiency notice? -21 . What is a notification? What is an amendment? How do I submit these changes to my Medical Device Establishment Licence (MDEL)? .
34. Will I receive a new copy of my Medical Device Establishment Licence (MDEL) each year?
A Medical Device Establishment Licence (MDEL) does not expire annually. An existing MDEL continues to be valid as long as a completed Annual Licence Review (ALR) application is received by Health Canada prior to April 1 of each year. You will only receive a revised copy of your MDEL if, as a result of the ALR process, there is a change that affects the content of your issued MDEL (e.g. changes to the class of medical devices, new activities, etc.).
Other questions
35. What do I do if my Medical Device Establishment Licence (MDEL) is cancelled?
Establishments that do not hold a valid Medical Device Establishment Licence (MDEL), including those whose licences are cancelled for not submitting an Annual Licence Review application before April 1, are not permitted to perform licensable activities.
In order to resume licensable activities, you will need to submit a completed application form and pay the fee once you've received an invoice. If the fee is paid and the application is found to be complete, a new MDEL number will be issued and you may resume licensable activities.
36. What do I do if my Medical Device Establishment Licence (MDEL) is suspended?
Establishments that have had their Medical Device Establishment Licence (MDEL) suspended are not permitted to perform licensable activities.
If you want to resume selling, importing or manufacturing medical devices after a suspension of your MDEL, you must submit a reinstatement application using FRM-0292.
To have your MDEL reinstated, your establishment will need to submit documentation with the application to demonstrate that the situation(s) that gave rise to the suspension has been corrected. The documentation can include, but is not limited to, an adequate Corrective and Preventive Action (CAPA) plan or a Corrective Action Plan (CAP). To verify the corrections, your establishment will be inspected by Health Canada before a licence decision is issued.
37. When will Health Canada "Pause the Clock" for a Medical Device Establishment Licence (MDEL) application?
Health Canada uses a "clock" to measure performance against the 120 calendar days performance standard.
- The clock starts on the date when Health Canada receives your complete application.
- When a deficiency is identified in your application, a deficiency notice is issued and the clock is paused.
- When Health Canada has received a response to a deficiency notice, the clock will be restarted.
General questions - Fees
38. Why does the Government of Canada charge for a Medical Device Establishment Licence (MDEL)?
For applications for a new MDEL and for the reinstatement of a suspended MDEL, the fee covers the examination of the application, as well as the cost of regulatory oversight for the fiscal year in which the application is received.
For an application for the ALR, the fee covers the examination of the ALR application, as well as the regulatory oversight for the subsequent fiscal year. This includes work related to inspections and establishment licensing.
39. Under what authority does Health Canada collect fees?
The Minister of Health has new fee setting authorities that grant the flexibility to fix and adjust fees in a timely way so that they better reflect actual costs. Fees will be charged as per the Fees in Respect of Drugs and Medical Devices Order.
40. What is the fee for a new Medical Device Establishment Licence (MDEL),a reinstated MDEL, or the Annual Licence Review of an existing MDEL?
The applicable fee for a new Medical Device Establishment Licence (MDEL), a reinstated MDEL or the Annual Licence Review of an existing MDEL, can be found on the Government of Canada's website: Fees for Examination of an Application for an Establishment Licence: Medical Devices.
41. How much will the Medical Device Establishment Licence (MDEL) fee go up every year?
The Medical Device Establishment Licence (MDEL) fee increases annually by an amount equivalent to the Consumer Price Index (CPI) from the previous year, beginning on April 1, 2021. Such annual adjustments are necessary to ensure that fees continue to keep pace with the impact of inflation on regulatory oversight costs. Each fall, Health Canada will publish a Notice of Intent in the Canada Gazettethat specifies the fees to be implemented the following April 1, and update the Health Canada website accordingly.
42. What if my licence was issued prior to April 1, 2020, and I am currently on deferred payment?
If you have applied for your Medical Device Establishment Licence (MDEL) prior to April 1, 2020 and have been granted a fee deferral, the payment of the applicable MDEL fee is deferred until the end of the first full calendar year after which the MDEL was issued. "Calendar year" means a period of 12 consecutive months commencing on January 1. At the end of the deferral period the applicant must pay all of the applicable fees. These will include the fees associated with the initial application and all subsequent annual licence reviews.
43. What will happen if the performance standard is missed?
For all applications filed on or after April 1, 2020, Health Canada's delivery of services against performance standards will be tracked per application. Most performance standards reflect the time to complete the review of the application, which is defined as the period from the date of receipt of the complete application to the date of approval or rejection of the application, not including any clock pauses or cost recovery holds.
In the event that a regulatory decision is not provided within the established performance standard, applicants will be credited 25% of the fee originally paid. Health Canada will notify the applicant accordingly following the processing of the application.
44. Is anyone exempted from fees?
Fees can be requested to be waived for applications filed by any branch or agency of the Government of Canada or of a province or territory.
To be considered for fee exemption, applicants must apply at the time of application directly on the application form.
45. Can my Medical Device Establishment Licence (MDEL) fee be reduced?
Fees can be requested to be reduced for Medical Device Establishment Licence (MDEL) applications filed by a small business.
To be considered for this type of fee mitigation, applicants must have applied for, and have been granted small business status by Health Canada, before submitting an MDEL application. Please visit Health Canada's Small business mitigation for drugs and medical devices: Small business fee reduction measures webpage for more information on how to apply as a small business or renew your existing status. Companies with valid small business status must indicate that they are a small business on the MDEL application form to qualify for the small business fee reduction.
46. Do I need to renew my small business status?
A company's small business status expires a year after registration. If you have previously registered as a small business with Health Canada, and still meet the definition as indicated above, you will need to ensure the status is renewed before submitting your Annual Licence Review (ALR) application. Please visit Health Canada's Small business for drugs and medical devices: Small business fee reduction measures webpage for more information on how to renew your existing status.
47. What is considered a Small Business?
A small business is defined in section 1 of the Fees in Respect of Drugs and Medical Devices Order as:
- any business, including its affiliates, that has fewer than 100 employees; OR
- has between $30,000 and $5 million in annual gross revenues.
48. What if Health Canada determines my company does not qualify as a small business after granting my company small business status?
Should Health Canada subsequently determine that the applicant does not qualify as a small business, the full fee is payable. In the event that the reduced amount had already been invoiced and paid, the difference between the full fee and the original fee is payable and a new invoice for the difference will be issued.
49. How do I calculate my gross revenue for the purposes of applying for Small Business?
To qualify for the small business mitigation under the Fees in Respect of Drugs and Medical Devices Order, companies must indicate their gross revenue (and that of their affiliates if applicable) resulting from all their sources of revenue/lines of business and not only the revenue generated by the activities under the Medical Device Establishment Licence (MDEL). Please note that the small business status applies to the company, not to the MDEL licence.
50. What is considered an affiliate for the small business status?
For the purposes of the new Fees in Respect of Drugs and Medical Devices Order that will come into force on April 1, 2020:
Affiliated companies are those that:
- one entity is affiliated with another entity if one of them is the subsidiary of the other or both are subsidiaries of the same entity or each of them is controlled by the same entity or individual;
- if two entities are affiliated with the same entity at the same time, they are deemed to be affiliated with each other; and
- an individual is affiliated with an entity if the individual controls the entity.
For the definition of a subsidiary entity, control and deemed affiliation, please consult section 1 of the new Fees in Respect of Drugs and Medical Devices Order.
51. If I have already applied for and received a Medical Device Establishment Licence (MDEL), am I still eligible for small business fee mitigation?
For existing Medical Device Establishment Licence (MDEL) holders, you must be registered as a small business before submitting your Annual Licence Review (ALR) application. Please visit Health Canada's Small business for drugs and medical devices: Small business fee reduction measures webpage for more information on how to apply for small business status.
52. Does the requirement to apply for small business status only apply to new applicants or does it also apply for the Medical Device Establishment Licence (MDEL) Annual Licence Review (ALR) as well?
To be eligible for the small business fee mitigation, applicants must apply for small business status before submitting their Medical Device Establishment Licence (MDEL) application. This applies to both new and existing MDEL holders.
For new applicants, they must apply for small business status prior to submitting their MDEL application.
For existing applicants, they must apply, or renew, for small business status prior to submitting their annual licence review application.
A company's small business status must be renewed yearly. Please visit Health Canada's Small business for drugs and medical devices: Small business fee reduction measures webpage for more information.
53. I am a new applicant. Do I need to pay when applying?
As of April 1, 2020, new applicants must pay the MDEL fee since fee deferrals will no longer be in effect. However, following the preliminary examination of a new Medical Device Establishment Licence (MDEL) application and upon determination that the application is accepted for further review, Health Canada will issue a notice to the applicant and an invoice for the applicable fee. Do not submit payment with your application. Payment is only required once the invoice from Health Canada is issued.
54. When do I pay?
Submit payment only after you receive an invoice from Health Canada.
55. What happens if I do not pay my fee?
Payment is due upon issuance of the invoice. Interest on overdue accounts begins to accrue 30 calendar days from the date of invoice issuance. It is the applicant's responsibility to ensure that payment is made on time.
In the event that the applicable fee is not paid in full within 30 days of the invoice date, the application will be placed on a cost-recovery hold, at which point all work associated with the application will stop. This hold will remain in place until the required payment is made. If the fee is not received within 30 days, the application may be rejected. In the case of annual licence review, the licence will be subject to cancellation.
Should Health Canada use this authority to stop the review of an application, the period of time where services are withheld does not count toward Health Canada's 120 calendar day performance standard.
56. Do I have to pay for a reinstated licence?
Yes. If you apply to have your licence reinstated after a suspension, you will pay the same fee as for a new MDEL or annual licence review application. The payment must be made after you receive an invoice from Health Canada.
57. If I am issued a new licence part way through the year, do I still have to pay the full fee?
Yes. You will have to pay the full fee as the MDEL fee is not prorated. Please note that for applications for a new MDEL, and for the reinstatement of a suspended MDEL, the fee covers the examination of the application, as well as the cost of regulatory oversight for the fiscal year in which the application is received.
The application period for the Annual Licence Review (ALR) runs from January to March, and the associated ALR fee is charged at the time of the ALR application. For an application for the ALR, the fee covers the examination of the ALR application, as well as the regulatory oversight for the subsequent fiscal year.
For example, for a new MDEL application received on Feb 1, 2021, a new application fee will be charged upon the receipt of the application. If the MDEL is granted before March 31, 2021, since the ALR runs from January to March, the new licence holder will also be required to file an ALR application for the subsequent fiscal year (April 1 2021 - March 31, 2022) and an additional MDEL fee will be payable upon receipt of the ALR application. This will result in two MDEL fees (i.e. one for the new MDEL application and one for the ALR application) being payable in the span of two months.
58. Will I be reimbursed if I withdraw my application for a Medical Device Establishment Licence (MDEL) or if my licence is cancelled?
No. The fee remains payable and will not be reimbursed. The fee is for the examination of an application. Therefore, if a company chooses to withdraw its application or if the licence is cancelled, Health Canada will not credit or refund any of the fees paid by the company, as these fees cover expenses already incurred.
If it is determined upon review that a device is not in fact a medical device, Health Canada will not refund the Medical Device Establishment Licence (MDEL) fee.
Note: it is the applicant's responsibility to determine that the device indicated on the application is considered a medical device in Canada and to obtain information on the class of the device. Please see question 7 . How should I confirm the classification of a device before applying for a Medical Device Establishment Licence? for instructions on confirming medical device classification.
Payment of fees
59. What methods are available for the payment of fees?
Fees may be paid by credit card (Visa, MasterCard, or American Express), cheques, money orders, international bank drafts, or bank wires.
For further instruction on the payment of fees, please see our guide on How to Pay Fees.
For further information on payments, contact Accounts Receivable at 1-800-815-0506 1-800-815-0506 or ar-cr@hc-sc.gc.ca.
60. Do I have to pay interest on an overdue account?
Yes. You have to pay interest on an overdue account. If full payment is not received within 30 days of the invoice date, interest will accrue. You will receive monthly invoices until the debt is cleared. Interest will not be waived once it is accrued. The fee plus any accrued interest will have to be paid.
Health Canada is committed to working with applicants whose accounts are in arrears, and encourages them to contact us as soon as possible to work out a monthly payment arrangement.
Applicants who have questions regarding their account may contact Accounts Receivable at:
61. What if I do not agree with the calculation of my fee?
If you do not agree with the calculation of your fees, you must pay the fee as per the invoice issued and file a request for reconsideration. This will ensure that the work on your application continues while the reconsideration is being conducted. Should the Cost Recovery Invoicing Unit (CRIU) determine that the fee was incorrectly calculated you will be credited or invoiced for the difference.
Please submit your request for reconsideration to:
Mail: Regulatory Operations and Enforcement Branch, Health Canada
Jeanne Mance Building
200 Eglantine Driveway - 4 th Floor
Ottawa, ON K1A 0K962. What happens in the case of an overpayment?
Overpayment of fees will be automatically credited to your account. A written request is required for a refund of a credit balance. In addition, you may request that we apply your credit balance to a full or partial payment of future fees. In this case, please attach to the submission / application a copy of the most recent statement indicating the account or client number, plus the amount of available credit.
The invoice number and your Medical Device Establishment Licence (MDEL) number must be included with all payments or it may lead to delays or errors.
For further instruction on how to submit payment, see our guide on How to Pay Fees.
63. Who should I contact if I have further questions?
For questions concerning a medical device establishment licence (MDEL) and the application process, contact:
Medical Devices Establishment Licence Unit
Regulatory Operations and Enforcement Branch, Health Canada
Jeanne Mance Building - 3 rd Floor
200 Eglantine Driveway
Ottawa, ON K1A 0K9
Address locator: 1903CFor questions about invoicing and fees for an MDEL, contact:
Cost Recovery Invoicing Unit
Regulatory Operations and Enforcement Branch, Health Canada
Jeanne Mance Building - 4 th Floor
200 Eglantine Driveway
Ottawa, ON K1A 0K9
Address locator: 1904C